- 0086-21-56469616
- 0086-18019205509
- minstar@minstargroup.com
- Language:English
- English
Your Location:Home >Products >Pharmaceutical >41753-43-9

Purity:99%
|
41753-43-9 Name |
|
|
Name |
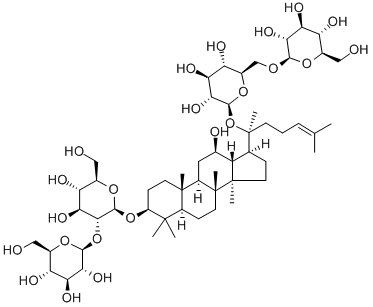
Ginsenoside Rb1 |
|
Synonym |
GINSENOSIDE GINSENOSIDE-RB1;GINSENOSIDE RB1;GINSENOSIDE RBL;(3beta,12beta)-20-[(6-o-beta-d-glucopyranosyl-beta-d-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl2-o-beta-d-glucopyranosyl-beta-d-glucopyranoside;2-O-beta-glucopyranosyl-(3beta,12beta)-20-[(6-O-beta-D-glucopyranosyl-beta-D-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl-beta-D-glucopyranoside;Ginsenoside Rb1 std.;ginsenoside-rb1from panax quinquefolium (american ginseng)root;Ginsenoside Rb1 (CAS# 41753-43-9) |
|
41753-43-9 Biological Activity |
|
|
Related Catalog |
Signaling Pathways >> Autophagy >> Autophagy Signaling Pathways >> Immunology/Inflammation >> IRAK Signaling Pathways >> Protein Tyrosine Kinase/RTK >> IRAK Signaling Pathways >> Autophagy >> Mitophagy Signaling Pathways >> Membrane Transporter/Ion Channel >> Na+/K+ ATPase Research Areas >> Inflammation/Immunology Natural Products >> Terpenoids and Glycosides |
|
Target |
Na+, K+-ATPase:6.3 μM (IC50) IRAK-1 p65 Autophagy Mitophagy |
Ginsenoside Rb1, the effective constituent of ginseng, has been demonstrated to play favorable roles in improving the immunity system. Ginsenoside Rb1 (or Ginsenoside Rb1 or GRb1 or GRb1) is a chemical compound belonging to the ginsenoside family. It is one of the primary constituents of traditional Chinese medicine, Ginseng. Reference standard in the analysis of herbal medicinal products. A major bioactive component of panax ginseng that promotes neurotransmitter release by modulating phosphorylation of synapsins through a cAMP-dependent protein kinase pathway. It has been reported to display immunostimulatory and anticancer effects.
InChI:InChI=1/C54H92O23/c1-23(2)10-9-14-54(8,77-48-44(69)40(65)37(62)29(74-48)22-70-46-42(67)38(63)34(59)26(19-55)71-46)24-11-16-53(7)33(24)25(58)18-31-51(5)15-13-32(50(3,4)30(51)12-17-52(31,53)6)75-49-45(41(66)36(61)28(21-57)73-49)76-47-43(68)39(64)35(60)27(20-56)72-47/h10,24-49,55-69H,9,11-22H2,1-8H3/t24-,25+,26+,27+,28+,29+,30-,31+,32-,33-,34+,35+,36+,37+,38-,39-,40-,41-,42+,43+,44+,45+,46?,47-,48-,49-,51-,52+,53+,54?/m0/s1
Six new protopanaxadiol-type ginsenosides, named ginsenosides Ra 4-Ra9 (1-6, resp.), along with 14 known dammarane-type triterpene saponins, were isolated from the root of Panax ginseng, one of the most important Chinese medicinal herbs.
Ginsenoside Rb1, a major bioactive component of Panax ginseng C. A. Mey., exerts beneficial effects on type 2 diabetes mellitus (T2DM), but its underlying mechanism is unclear. In summary, our study showed that Rb1 improved T2DM by regulating hepatic glucose metabolism. More importantly, our experiments first demonstrated that STAT3 is an important regulator in Rb1-ameliorated glucose disorder in the liver of T2DM mice.
![(20S)-3-O-{β-D-6-O-[(E)-but-2-enoyl]glucopyranosyl-(1->2)-β-D-glucopyranosyl}-20-O-[β-D-glucopyranosyl-(1->6)-β-D-glucopyranosyl]protopanaxadiol](/upload/2023/1/afb9d925-96c5-431a-b30f-80a92ad7d13a.png)
(20S)-3-O-{β-D-6-O-[(E)-but-2-enoyl]glucopyranosyl-(1->2)-β-D-glucopyranosyl}-20-O-[β-D-glucopyranosyl-(1->6)-β-D-glucopyranosyl]protopanaxadiol


Ginsenoside Rb1
| Conditions | Yield |
|---|---|
|
With sodium methylate; In methanol; at 20 ℃; for 12h;
|

malonyl-ginsenoside Rb1


malonic acid


ginsenoside Rb1
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; In methanol; at 22 ℃; for 0.5h; Product distribution; malonic acid determined as its methyl ester by GLC;
|

(20S)-3-O-{β-D-6-O-[(E)-but-2-enoyl]glucopyranosyl-(1->2)-β-D-glucopyranosyl}-20-O-[β-D-glucopyranosyl-(1->6)-β-D-glucopyranosyl]protopanaxadiol

malonyl-ginsenoside Rb1

(3β,12β,20R/20S)-12,20-dihydroxydammar-24-en-3-yl 2-O-(6-O-acetyl-β-D-glucopyranosyl)-β-D-glucopyranoside

20(R)-ginsenoside Rg3

ginsenoside Rg3

ginsenoside Rg5
CAS:885523-08-0
CAS:517920-73-9
CAS:960404-48-2