- 0086-21-56469616
- 0086-18019205509
- minstar@minstargroup.com
- Language:English
- English


Appearance:white to off-white crystalline powder or needles
Purity:99%
|
768-95-6 Name |
|
|
Name |
1-Adamantanol |
|
Synonym |
TRICYCLO[3.3.1.1(3,7)]DECAN-1-OL;TRICYCLO[3.3.1.1]DECAN-1-OL;1-HYDROXYDIAMANTANE;1-HYDROXYADAMANTANE;1-AD-OH;1-ADA-OH;1-ADAMANTANOL;1-Adamantol |
|
768-95-6 Chemical & Physical Properties |
|
|
Melting point |
247 °C (subl.)(lit.) |
|
Boiling point |
245.8±8.0 °C at 760 mmHg |
|
Density |
1.2±0.1 g/cm3 |
|
Molecular Formula |
C10H16O |
|
Molecular Weight |
152.233 |
|
Flash Point |
101.1±10.9 °C |
|
PSA |
20.23000 |
|
LogP |
2.16 |
|
Exact Mass |
152.120117 |
|
Vapour Pressure |
0.0±1.1 mmHg at 25°C |
|
Index of Refraction |
1.581 |
|
Storage condition |
2-8°C |
1-adamantanol is also known as 1-hydroxide adamantane, 1-tricyclo [3.3.1.1 (3.7)] decyl alcohol. At room temperature, it is white crystalline powder with the melting point higher than 240 ℃. It is soluble in organic solvents and insoluble in water with sublimation property. It can be used for the manufacture of synthetic adamantane derivatives and adapalene. 1-Adamantanol was used in the preparation of 1,3-adamantanediol. It is used in the manufacture of synthetic adamantane derivatives and adapalene. If 2-adamantanol is a suspected impurity, then dissolve the substance (10g) in acetone (100mL) and add Jones's reagent [CrO3 (10.3g) in H2O (30mL)], then conc H2SO4 (8.7mL) is added dropwise (turns green in colour) until excess reagent is present (slight red colour).
InChI:InChI=1/C10H16O/c11-10-4-7-1-8(5-10)3-9(2-7)6-10/h7-9,11H,1-6H2
The enthalpies of combustion and of sublimation four mono-oxygenated diamantanes have been determined. Comparisons are made between the experimental gas-phase enthalpies of formation and those obtained by molecular mechanics (empirical force field) calculations based on the Allinger MM1 force field model.
Aprotic diazotization of endo-7-aminomethylbicyclo[3,3,1]nonan-3-one yields protoadamantan-4-one and 3-methylbicyclo[3,3,1]non-2-en-7-one; the latter was converted into adamantan-1-ol on catalytic hydrogenation with Pd-C.
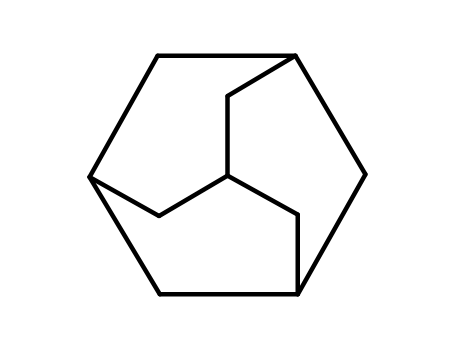
adamantane

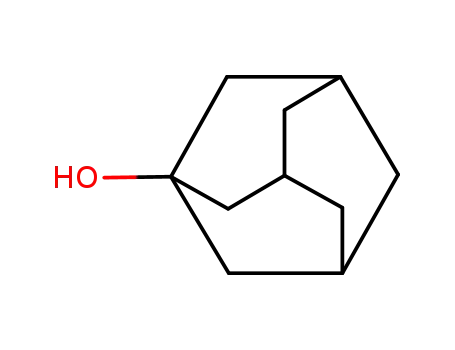
1-adamanthanol

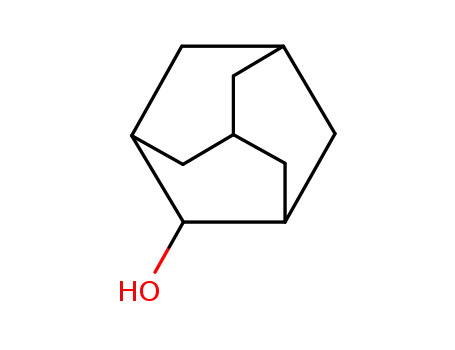
1-adamantanol

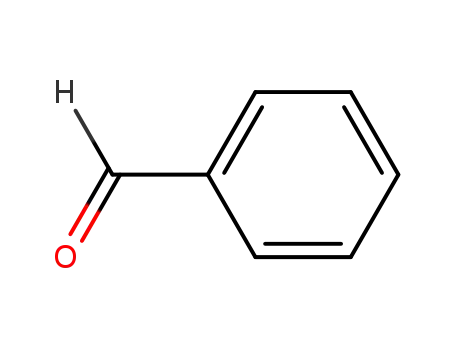
benzaldehyde

benzyl alcohol
| Conditions | Yield |
|---|---|
|
With meso-tetraphenylporphyrin iron(III) chloride; peroxyphenylacetic acid; In benzene; at 25 ℃; for 0.166667h; Product distribution; other alkane; other peroxy acid; also var. iron(III) porphyrin-deriv. - ligand complexes;
|
61% 7% |

acetonitrile

trans-N,N'-bis(1-adamantyl)diazene

1,1'-Biadamantane


1-adamanthanol

N-(1-adamantyl)acetamide

1-acetyladamantane
| Conditions | Yield |
|---|---|
|
With thianthrenium perchlorate; Further byproducts given; Ambient temperature;
|
90% 2.5% 0.6% 5.5% |
1-Adamantyl bromide
pyridine

adamantane
methanol
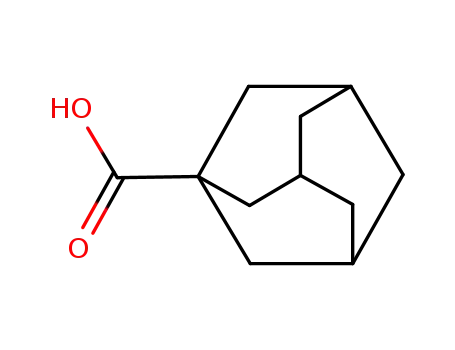
1-Adamantanecarboxylic acid
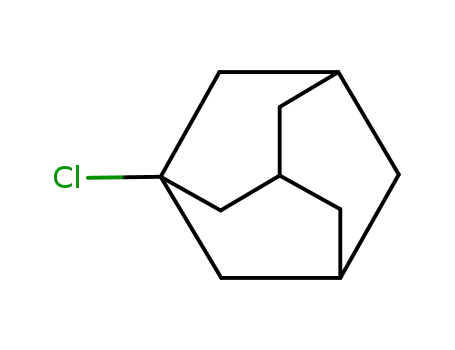
1-chloroadamantane

N-(1-adamantyl)acetamide
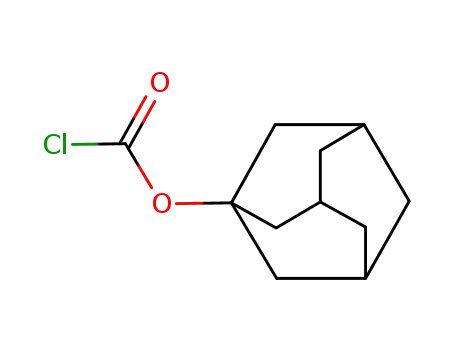
1-adamantyl chloroformate
CAS:885523-08-0
CAS:517920-73-9
CAS:1314981-48-0
CAS:25108-33-2