- 0086-21-56469616
- 0086-18019205509
- minstar@minstargroup.com
- Language:English
- English
Your Location:Home >Products >Inorganics >2094-72-6

Appearance:white to almost white crystalline solid
Purity:99%
|
2094-72-6 Name |
|
|
Name |
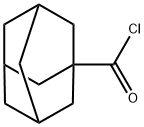
1-Adamantanecarbonyl chloride |
|
Synonym |
1-ADAMANTANECARBONYL CHLORIDE;1-ADAMANTANECARBOXYLIC ACID CHLORIDE;1-ADAMANTANECARBOXYLIC ACID CHLOROANHYDRIDE;ADAMANTANE-1-CARBONYL CHLORIDE;AKOS BC-0659;TRICYCLO[3.3.1.1]DECANE-1-CARBOXYLIC ACID CHLORIDE;1-Adamantanecarbony chloride;38. 1-adamantanecarbonyl chloride |
|
Chemical & Physical Properties |
|
|
Melting point |
49-51 °C(lit.) |
|
Boiling point |
270.6±0.0 °C at 760 mmHg |
|
Density |
1.2±0.1 g/cm3 |
|
Molecular Formula |
C11H15ClO |
|
Molecular Weight |
198.689 |
|
Flash Point |
139.0±8.3 °C |
|
PSA |
17.07000 |
|
LogP |
3.34 |
|
Exact Mass |
198.081146 |
|
Vapour Pressure |
0.0±0.5 mmHg at 25°C |
|
Index of Refraction |
1.556 |
|
Storage condition |
2-8°C |
|
Water Solubility |
may decompose |
1-Adamantanecarbonyl chloride was used in the preparation of efficient amine-modified oligodeoxynucleotides for fabrication of DNA microarrays. The hydroxyl groups of NDs first reacted with 1, 1-adamantanecarbonyl chloride to obtain ND-Ad, IUdR was derivatized with either 1-adamantanecarbonyl chloride or 4-(1-adamantyl-carbamoyl)butanoic acid, to prepare 5’-O-(1-adamantoyl)-5-iodo-2’-deoxyuridine 1 and 5’-O-(4-(1-adamantylcarbamoyl)butoyl)-5-iodo-2’-deoxy-uridine 4, respectively. 1-adamantanecarbonyl chloride is the most common and is available commercially in bulk quantities.
InChI:InChI=1/C11H15ClO/c12-10(13)11-4-7-1-8(5-11)3-9(2-7)6-11/h7-9H,1-6H2
A cyclic voltammogram for reduction of 1‐adamantanecarbonyl chloride at a glassy carbon or hanging mercury drop electrode in acetonitrile containing 0.10M tetraethylammonium perchlorate displays two irreversible cathodic waves, we conclude that 1‐adamantanecarbonyl chloride undergoes one‐electron reduction to an acyl radical, which accepts a hydrogen (deuterium) atom from the solvent to afford the aldehyde.
The relative contributions of the main intermolecular contacts as well as the enrichment ratios derived from the Hirshfeld surface analysis establish the 1-acyl thiourea synthon to be a widespread contributor.
Chlorin e6 (Ce6) was purchased from Frontiersci. Nsingle bondHydroxysulfosuccinimide (NHS), 1-Adamantanecarbonyl chloride, N-Boc-ethylenediamine, N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide (EDC), cysteamine (Cys), dithiothreitol (DTT), and 4-dimethylaminopyridine (DMAP) were provided.

adamontoyltris(trimethylsilyl)germane


tris(trimethylsilyl)germyl chloride


{(CH3)3Si}2Ge{C(OSi(CH3)3)C10H15}


chloroform


1-Adamantanecarbonyl chloride
| Conditions | Yield |
|---|---|
|
In tetrachloromethane; byproducts: Me3SiCl, hexachloroethane; Irradiation (UV/VIS); NMR tubes contg. the acylgermane/CCl4 sealed under vac., irradiated (two 100W Hg lamps, λ>360 nm) for up to 3h, with cooling to -15°C), soln. remained colourless; not isolated, detected by NMR-spect.;
|
>65 >99 <1 0% |

1-Adamantyl bromide


1-Adamantanecarbonyl chloride
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 1.) magnesium, 1,2-dibromoethane / 1.) diethyl ether, 35 deg C
2: 90 percent / SOCl2 / 1 h / Heating
With thionyl chloride; magnesium; ethylene dibromide;
|
|
|
Multi-step reaction with 2 steps
1: sulfuric acid / hexane / 5 h / Cooling with ice
2: thionyl chloride / benzene / 5 h / 80 °C / Reflux
With thionyl chloride; sulfuric acid; In hexane; benzene;
|
|
|
Multi-step reaction with 2 steps
1.1: magnesium; iodine / diethyl ether / 2 h / Inert atmosphere
1.2: 3.5 h / Inert atmosphere
2.1: thionyl chloride / 1 h / 80 °C
With thionyl chloride; iodine; magnesium; In diethyl ether;
|
|
|
Multi-step reaction with 2 steps
1: sulfuric acid / tetrahydrofuran / 3 h / 5 - 10 °C
2: thionyl chloride / 2 h / 80 °C
With thionyl chloride; sulfuric acid; In tetrahydrofuran;
|
|
|
Multi-step reaction with 2 steps
1: sulfuric acid / tetrahydrofuran / 3 h / 5 - 10 °C / Green chemistry
2: thionyl chloride / 2 h / 80 °C / Green chemistry
With thionyl chloride; sulfuric acid; In tetrahydrofuran;
|
|
|
Multi-step reaction with 2 steps
1: sulfuric acid / hexane / 4 h / 5 - 10 °C
2: thionyl chloride / 1 h / 80 °C
With thionyl chloride; sulfuric acid; In hexane;
|
|
|
Multi-step reaction with 2 steps
1: sulfuric acid / hexane / 4 h / 5 - 10 °C
2: thionyl chloride / 1 h / 80 °C
With thionyl chloride; sulfuric acid; In hexane;
|

1-Adamantanecarboxylic acid

1-Adamantyl bromide

adamontoyltris(trimethylsilyl)germane

adamantane

(adamant-1-yl)-acetic acid

((morphonyl)tricyclo[3.3.1.13,7]dec-1-yl)methanone

N-(benzo[d]thiazol-2-yl)adamantane-1-carboxamide

1-(adamantan-1-yl)propan-1-one
CAS:5451-09-2
CAS:764-85-2
CAS:1094-61-7
CAS:677-22-5